Declaration of handling of animal foodstuffs and health approval
Verified 30 October 2025 - Directorate of Legal and Administrative Information (Prime Minister)
Any establishment that product, manipulate or transformed food of animal origin intended for human consumption must declare it if it sells it only to consumers. In case of sales to other professionals, it is necessary to apply for a health approval.
Declaration: sale to consumers
General principle
The declaration shall apply to any operator who carries out any of the stages of production, processing and distribution of food of animal origin intended for the production of human consumption.
Any operator who product, transformed, manipulate, distributed, sells or warehouse food of animal origin is required to declare each of the establishments for which it is responsible, as well as activities that take place there. Also covered are products containing food of animal origin (e.g. all products containing eggs, milk, etc.).
The declaration is mandatory only for sales or distribution activities to final consumers and not for sales to other establishments. For sale to professionals, a health approval is necessary.
FYI
The declaration must be renewed at each change operator, address or nature of activity.
Examples of establishments concerned
The establishments concerned are for example:
- Restaurant, company restaurant, collective catering
- Food shops: butcher, delicatessen, cheese shop, fish shop, caterer, ice cream parlor, pastry chef, chocolatier, etc.
- Retail stores: grocery store, frozen food store, etc.
- Large food distribution: for example, a processing laboratory in a supermarket
- Compound product preparation activity (containing processed products of animal and plant origin)
- Food delivery or carrying meals at home
- Storage of food of animal origin
- Farm production, e.g. eggs, milk, butter, cheese, or bread produced and sold on the farm, including the slaughter of poultry and lagomorphs (rabbits and hares) on the farm
- Fishing for fish and shellfish (fresh water or at sea, by vessels)
- Logistics center for landing of fishery products
- Platform for the bursting of fishery products
- Carrier of animals or animal products
- Food distribution charities (any free distribution)
Activities not covered by the declaration
The declaration does not cover the following activities:
- Primary production : rearing animals until slaughter, hunting, egg production until packaging, milk production on the farm without packaging. It should be noted that some of these activities require authorization.
- Activities carried out within the framework domestic (preparing meals for family and loved ones)
- Activities requiring health approval (sale between professionals). In this case, approval is sufficient.
Activities subject to specific authorization (eggs, milk)
Some sales of primary productions do not require the declaration of handling of animal foodstuffs, but they are subject to authorization.
This is the case with the sale of raw milk andeggs, as such, not packaged, directly or through a point of sale, at final consumer .
The authorization shall be issued without limitation of duration or quantity delivered to the consumer. The trader must deliver his milk or eggs to the final consumer at the production site (e.g. farm sale), at a local public market (within 80 km of the farm) or by peddling in the production area.
If an operator holds a health license for sale to professionals, he does not need an authorization. THEsanitary approval is sufficient.
Please note
There is also a specific authorization for the boning of beef over 30 months containing vertebral bone. This request must be made directly to the department (DDPP: titleContent) the location of the establishment (each department having its own form).
How to apply for an authorization for the direct sale of unpackaged raw milk?
The application for authorization for the sale of raw milk is done either online or by mail.
Online
The following online service allows you to make the authorization request:
Applying for an authorization to produce and place on the market raw milk
By mail
The paper form must be downloaded, completed and printed. It must be sent to the Departmental Directorate of Population Protection (DDPP) of the establishment's location.
Application for authorization to produce and place on the market raw milk (paper)
How to apply for an authorization for the direct sale of fresh unpackaged eggs?
As regards egg producers, only holders of less than 250 hens laying machines.
Eggs can thus be sold without having been graded by an egg packing center.
The application for authorization for the sale of fresh eggs is done either online or by mail.
Online
The following online service allows you to make the authorization request:
By mail
The paper form must be downloaded, completed and printed. It must be sent to the Departmental Directorate of Population Protection (DDPP) and the Regional Directorate of Food, Agriculture and Forestry (DRAAF) of the establishment's location.
The trader must make the declaration before the activity starts.
It is done either online or by post via a form.
Online
To complete the online procedure, you must use the following service:
By mail
The form must be downloaded, completed and printed. It must be sent to the Departmental Directorate for Population Protection (DDPP) for establishments located in metropolitan France, or to the Directorate of Agriculture, Food and Forestry for the Overseas Departments.
Approval: sales to professionals
It is any operator who prepare, transformed, manipulate or warehouse products of animal origin or foodstuffs containing such products (e.g. meat, cold cuts, meat-based dishes) and which marketed fromother professionals.
Please note
Establishments that sell directly to final consumer are not concerned by the health approval. A declaration of manipulation is sufficient.
The establishments concerned by therequirement for health approval are as follows:
- Slaughterhouse or cutting plant, except for on-farm slaughter of poultry, hares and rabbits (one declaration is sufficient)
- Cheese manufacturer or milk processing establishment
- Manufacturer of minced meat, meat products or preserved products of animal origin
- Freezer vessel and factory vessel, including crustacean and mollusk cooking vessel
- Auction market or tidal hall
- Wholesale or wholesale market for food products containing products of animal origin
- Fresh produce packer: milk collection center, egg packing center in particular
- A food establishment that sells ready meals to intermediaries.
The list of EC approved establishments in terms of health security (who hold this health approval) is available online.
Establishments subject to health approval
Health approval procedures
Obligation to declare and approve establishments producing or using foodstuffs: Article 6
Registration and approval of establishments: Article 4
Health approval is not not required for the following activities:
- Primary production (culture/breeding): rearing animals until slaughter, hunting, producing honey, eggs until they are packaged, raw milk on the farm without packaging, picking snails, for example. It should be noted that, for the direct sale to consumers of raw milk and eggs, a specific authorization is required.
- Transport of animals or products of animal origin
- Manufacture of composite products by assembling foodstuffs of plant origin (bread, semolina, vegetables in particular) and purchased foodstuffs already processed (ham, smoked salmon, cheese). These include pizza, quiche, sandwich, paella.
- Retail sale or direct delivery to consumers (subject to the obligation to manipulation declaration of food of animal origin)
- Storage of products of animal origin at the place of production.
Please note
It is recommended in all cases of inquire before with local services (DDPP or DRAAF), in order to check whether or not the proposed activity is subject to health approval. The official bulletin of the Ministry of Agriculture also gives details of the approval.
The services from which the operator can obtain information are as follows:
Who shall I contact
Or for overseas:
Who shall I contact
General case
Overseas
The retail may benefit from a derogation from the authorization.
A retail operator supplying food of animal origin to another local retail establishment (e.g. a butcher supplying meat to a caterer) may apply for an exemption from health approval.
The distance as the crow flies between the establishment of the beneficiary of the derogation and the retail trade supplied must not exceed 80 km (distance may be extended up to 200 km by prefectural decision, due to particular geographical constraints).
The waiver is possible if the company provides a limited quantity per week of certain specified products.
Category of products | Maximum quantity that can be divested (per week) | |
|---|---|---|
if it is less than 30% of the total production of the establishment | if it represents more than 30% of the total production of the establishment | |
Heat-treated milks | 800 liters | 250 liters |
Dairy products | 250 kg | 100 kg |
Fresh meat from butchery * | 800 kg | 250 kg |
Meat products, prepared meals, meat preparations, fresh meat of species other than butchery * | 250 kg | 100 kg |
Products made from egg shell and/or raw milk which have undergone a sanitizing treatment other than dairy products | 250 kg | 100 kg |
Unprocessed fishery products (chilled or frozen, prepared or whole) | 250 kg | 100 kg |
Processed fishery products (salted, smoked, cooked) | 250 kg | 100 kg |
Snails (whole, prepared or processed) | 100 kg | 30 kg |
* except minced meat
The quantities indicated are cumulative between products belonging to different categories (e.g. possible delivery of 800 kg of beef + 250 kg of cold cuts).
Please note
In the event that a derogation from the health approval is granted, consideration should be given to making a declaration.
The request for exemption can be made either online or by post.
Online
In order to apply for the exemption from the online health authorization, the operator must use the following service:
By mail
To apply for a health authorization waiver, the operator must download, print and complete the following form:
Derogation from the health approval requirement
In metropolitan France:
Who shall I contact
Overseas:
Who shall I contact
Quantities of foodstuffs for possible derogations: Annexes 3 and 4
The application must be filed before the start of the activity.
The health approval is issued by the prefecture to an establishment for a given activity. If the company has several different activities, several approvals must be requested.
Approval shall be granted after the visit of a inspector the services of the Ministry of Agriculture.
The purpose of this visit is to check the premises, the equipment, the proper functioning of the site and the application of the PMS or health control plan (good hygiene practices, withdrawal/recall procedures in case of health alert, traceability system in particular).
The applicant may consult the guide to good hygiene practices (GBPH) relating to its activity.
Please note
Some request type folders the accreditation documents are available on the website of the Ministry of Agriculture in the My steps section (examples: dairy farmer's workshop, creamer-cheese-ripener, delicatessen).
The list of documents to be provided with this request (whether it is the online or the postal approach) is available online.
FYI
The file with all the documents to be provided must be constituted and scanned before performing the step.
The application for health approval is made either online or by post.
By mail
The applicant must download, print and complete the following form. It must be sent to the Departmental Directorate for Population Protection (DDPP) for establishments located in metropolitan France, or to the Directorate of Agriculture, Food and Forestry for the Overseas Departments.
Procedure and documents to be provided for the application for health approval
Foodstuffs and activities concerned
Refusal is possible.
It can occur either as a result offile review request, or after a health inspection visit.
If the application file remains unanswered for more than 2 months, the case is considered rejected. The operator shall receive a acknowledgment of receipt from the date on which the administration receives the file. If documents are missing from the file or are non-compliant, she informs him and he must complete or correct it.
Operator receives approval conditional, which becomes final only after the visit of the health services. Approval may be refused if certain major non-conformities detected during the inspection are not corrected.
Please note
It is recommended to inquire with local services (DDPP: titleContent or Draaf: titleContent) in order to verify whether or not the proposed activity is subject to health approval. The official bulletin of the Ministry of Agriculture (DGAL/SDSSA/2022-349 25/04/2022) give details of the control visit and the grounds for refusal.
Grounds for refusal or withdrawal: Article 6(1)
Details on the grounds for refusal or withdrawal
In some cases, the initial approval is no longer valid and the application has to be renewed.
The validity of the approval is not limited in time but must be renewed in case of change in the activity.
These are the following:
- Handling a new product category not in the original list
- Exercise of a new activity not in the original list
- Significant alteration of premises, their layout, equipment or use
The renewal of the application shall be carried out in the same way as an initial application for approval.
The documents accompanying the application must again be provided in their modified, updated version.
The request can be made either online or by post.
Online
To apply for online license renewal, the operator must use the following service:
By mail
To apply for the renewal of the health authorization, the operator must download, print and fill out the following form:
In metropolitan France:
Who shall I contact
Overseas:
Who shall I contact
In the event of non-compliance with infrastructure, equipment or operational requirements, or non-compliance of a product endangering the health of the consumer, the approval may be suspended provisionally or removed.
Example :
In the event of deterioration of the health conditions of the establishment, the inspection (during an inspection) must suspend the approval until the conformity has been repaired.
Following a withdrawal or suspension of the health approval, the establishment can no longer manufacture or place its products on the market.
Grounds for refusal or withdrawal: Article 6(1)
Details on the grounds for refusal or withdrawal
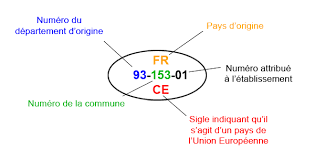
All products of animal origin from an approved establishment must be identifiable by a identification mark, also called health stamp or health mark.
The identification mark is oval in shape.
It shall include the following:
- Country Code
- Department Number
- Number of the municipality
- Order number in the municipality granted to the establishment
- CE symbol indicating that it is an EU countryEU: titleContent.
This brand is called:
- Health mark on carcasses, half-carcasses and quarters in slaughterhouses of animals for slaughter
- Sanitary stamp on other products.
If the approved establishment produces both food subject to approval and food not subject to approval, it may affix the same identification mark to both types of food.
Please note
It should not be confused with the EMB code (also oval) that gives information about the packaging.
Food marking: Article 5 of the Regulation
Aquaculture refers to all activities of animal production or vegetable in aquatic environment.
To be authorized to place on the market aquaculture animals, processing and aquaculture establishments must obtain animal health approval.
Each site of an aquaculture operation (farm or shellfish farming area) must be the subject of a separate application.
The person in charge of the aquaculture holding and mollusk farming areas shall carry out the following procedures:
- Keep a record of all movements in and out of aquaculture animals, indicating their origin, destination, number or weight and size
- Record the mortality observed in each epidemiological segment in relation to the type of production
- Implement good animal health practices to prevent the introduction and spread of diseases
- Collect the results of the animal health surveillance plan approved by the prefect. This collection makes it possible, on the basis of the analysis of the animal health risks for each type of production, to detect any unexplained and significant increase in mortality.
The operator must complete the following form:
Application for animal health approval for aquaculture
The form must be downloaded, printed, completed and sent to the Departmental Directorate of Population Protection (DDPP) which now includes veterinary services.
Who shall I contact
Who can help me?
Find who can answer your questions in your region
Requirement for health approval
Commodities, activities and establishments concerned by the declaration and approval
Declaration of the establishments concerned
Conditions for health approval and declaration of handling of foodstuffs
Attachments for all types of application for approval in Annex II of the Order
Non-approved poultry and lagomorphs (hares and rabbits) slaughter establishments
Details on the grounds for refusal and withdrawal, control visit
Obligation to declare and approve establishments producing or using foodstuffs: Article 6
Registration and approval of establishments: Article 4
Online service
Ministry of Agriculture
Ministry of Agriculture
Ministry of Agriculture
Ministry of Agriculture


